Don't forget the caregivers
in health technology assessments for Alzheimer's
Our viewpoint
10 August 2023
Why HTAs should recognise caregiver burden if patients are to benefit from revolutionary Alzheimer's drugs
A new era for Alzheimer’s disease
The recent FDA approval of lecanemab (Leqembi), and promising results from donanemab, represent a landmark moment in the history of Alzheimer’s disease. These two compounds are the first true disease-modifying therapies to treat the disease rather than the symptoms. Whilst symptomatic treatments may help to reduce or control cognitive or behavioral symptoms, they have no impact on disease progression. Lecanemab and donanemab, on the other hand, work to remove amyloid, a key pathological hallmark of Alzheimer’s disease, and both drugs have been shown in randomized controlled trials to have slowed the progression of disease amongst patients with mild cognitive impairment.
Let’s talk about price
After more than 500 failed Alzheimer’s disease clinical trials in the last two decades (Kim et al., 2022), innovative therapeutics that deliver a step change deserve a price. However, that price should reflect the total value that these medicines bring patients, families and society.
Lecanemab has a list price of $26,500 per year in the United States (US), and whilst Eli Lilly has yet to set a price for donanemab, it is likely to be in a similar range. The Institute for Clinical and Economic Review (ICER) in the US assessed lecanemab’s value at this price and calculated an incremental cost-effectiveness ratio (the price of net benefits of the drug) of $204,000 per equal value life year gained. This suggests that treatment with lecanemab would not be cost-effective (or provide enough value) as it lies above a potential cost-effectiveness threshold of $200,000 (Lin et al., 2023).
Impact of Alzheimer’s disease on caregivers
A US study estimated that 11.2 million family caregivers provided an estimated 15.3 billion hours of care to people with Alzheimer’s disease in 2020. Using minimum wages to calculate opportunity costs, the net economic value of this unpaid caregiving in 2020 was estimated to be USD 256.7 billion (Park, Marcum and Garrison, 2021).
Caregiving for someone with Alzheimer’s disease may lead to a decline in the caregiver's emotional or mental wellbeing, and increase the likelihood of them experiencing anxiety, depression, and disturbed sleep (Ory et al., 1999; Gaugler et al., 2005; Karg et al., 2018; Montgomery et al., 2018). This impact on quality of life for caregivers was estimated to cost an additional USD 57 billion in 2020 (Park, Marcum and Garrison, 2021).
We now know that introducing treatments for Alzheimer’s disease has an impact on caregiver burden. Recent results from the Clarity-AD trial showed that use of lecanemab slowed worsening of caregiver burden by 38%. (Cohen et al., 2023).
It is therefore important to include benefits to caregivers when calculating the cost-effectiveness of lecanemab. Taking caregiver burden into account, ICER calculated an incremental cost-effectiveness ratio of $183,000 per equal value life year gained, lower than a potential US cost-effectiveness threshold of $200,000. In this case, a $26,500 price tag would be value based (Lin et al., 2023).
A review of prior cost-effectiveness evaluations in Alzheimer’s disease further supports this finding, showing that the inclusion of caregiver benefits reduced incremental cost-effectiveness ratios in approximately 45% of evaluations. Furthermore, in 33% of cases, the reduction brought the incremental cost-effectiveness ratio under cost-effectiveness thresholds, implying that the inclusion of caregiver benefits might affect reimbursement decisions (Lin et al., 2019).
Are patients in Europe going to miss out?
ICER does not have a formal role for recommending reimbursement of medications in the US, unlike Health Technology Assessment (HTA) agencies in Europe. But HTA submissions highlighting caregiver benefits are infrequent, and HTA agencies seem to give caregiver benefit minimal importance, with little to no discernible influence on reimbursement determinations (Pennington 2020; Bauer et al., 2020). Among HTA submissions that did include caregiver benefits, the methods used to calculate those benefits varied. Nevertheless, in each scenario where caregiver benefits were included, incremental cost-effectiveness ratios decreased (Pennington, 2020).
Despite the significant potential implications of including caregiver benefits in cost-effectiveness calculations, a recent examination of global HTA policies revealed limited guidance from HTA agencies concerning assessment of the caregiver perspective (Pennington et al., 2022). This may explain why submissions rarely include this information and, if done, use different methods.
If caregiver benefits are not valued in assessments, HTA agencies may undervalue treatments for Alzheimer’s disease and therefore restrict their reimbursement, meaning that people in Europe with Alzheimer’s disease may not be able to access these treatments.
What should be done?
Existing frameworks for HTAs must be updated to include the additional elements of value that novel therapies for Alzheimer’s disease may bring, such as caregiver benefits (defined under family spillover in the ISPOR Value Flower, below), insurance value, severity of disease, value of hope, real option value and scientific spillovers.
To include the caregiver perspective in HTA submissions with any consistency, some challenges need to be addressed. These include defining the number of caregivers impacted, how best to capture caregiver quality of life, and how to avoid ‘double counting’ if caregivers complete quality of life questionnaires for both themselves and the person they care for. Congruent methodological guidance from all HTA agencies regarding these issues is needed to enable consistency in decision making. Whilst caregiver benefits can be modelled through a cost-effectiveness HTA framework, for countries that use a more clinical effectiveness framework, e.g. Germany, caregiver benefits need to be given equal weight. This is essential to prevent geographical inequalities in patient access to these ground-breaking treatments.
The ISPOR value flower
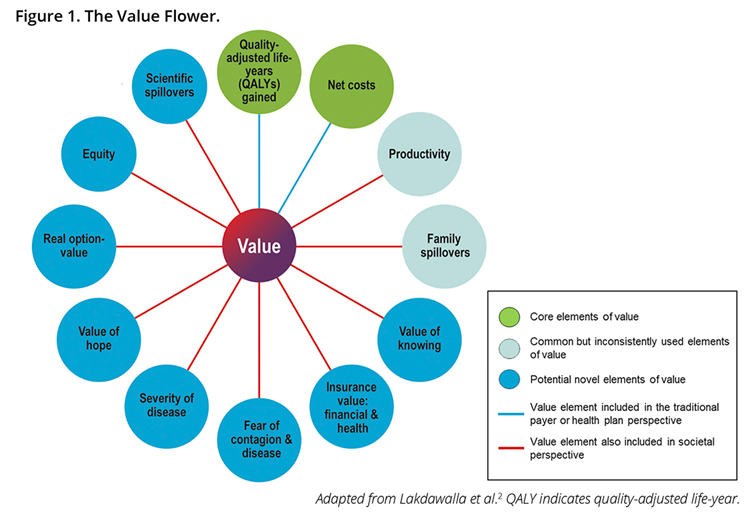
Each ‘petal’ reflects a different element of value:
- Quality adjusted life years - a common health outcome metric calculated by: Life years gained x Quality of life weighting;
- Net costs –the cost savings the intervention brings net of the cost of implementation;
- Productivity – often considered as loss of ability to work;
- Family spillovers – how family members are affected by a patient’s condition;
- Value of knowing – the additional value of being able to predict who will respond well to a new treatment;
- Insurance value – the value to healthy individuals of a new technology that protects them from the physical and financial burden of ill-health;
- Fear of contagion and disease – the value in reducing fear of transmission of disease;
- Severity of disease – a greater value may be derived from an intervention that treats more severe conditions;
- Value of hope – the value that some may place on a treatment being beneficial where the treatment has uncertain effects and cannot be predicted beforehand;
- Real option value – the value of extending life to benefit from future technologies and interventions;
- Equity – costs and QALYs are often viewed as averages per person that do not take into account equity of outcomes across different population groups (e.g. by race or socioeconomic status). There has been a suggestion that equity could be factored into economic evaluations through distributional cost effectiveness analysis;
- Scientific spillovers – value when one innovation eases the development of other interventions.
See our blog exploring additional elements of value for more information.

